1. First look at amorphous carbon: what is it?
1.1 The difference between graphite system and amorphous carbon
Graphite mainly has ABAB stacked hexagonal structure (2H or phase) and ABCABC stacked rhombic structure (3R or phase). The two phases of graphite can be converted to each other. Processes such as mechanical treatment can increase the phase composition ratio in graphite. Annealing at high temperature A more thermodynamically stable phase would result. Graphite has become the most common negative electrode material for commercial lithium-ion batteries due to its long-range ordered stacking structure, good electrical conductivity, high specific capacity, and good cycle performance. The raw materials are mainly asphalt, petroleum, etc. Coke and natural graphite, the interlayer spacing is about 0.335 to 0.34nm. Amorphous carbon mainly includes hard carbon and soft carbon, usually composed of randomly distributed graphitized microstructures, twisted graphene nanosheets and pores between the above microstructures, lacking an ordered stacking structure. Among them, soft carbon, also known as graphitizable carbon, will transform into graphite at a high temperature above 2800°C. The crystals are similar to graphite but have a lower degree of order. The short-range ordered graphitized microcrystalline structure is conducive to intercalation and storage of sodium. Hard carbon is non-graphitizable carbon, which is difficult to graphitize even when heated to 2800°C. Its structure is highly disordered and its oxidation-reduction potential is low. It is considered to be an ideal anode material for sodium-ion batteries.
1.2. Selection of carbon anode materials for sodium-ion batteries
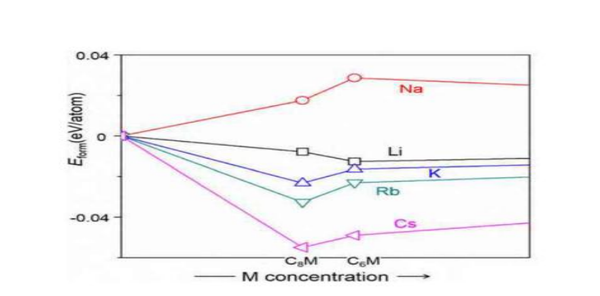
Although graphite itself has a good lithium storage specific capacity (372mAh/g), it also plays an important role in the field of lithium-ion batteries, but due to the large radius of sodium ions, it hinders the insertion and extraction of sodium ions during charging and discharging. Graphite cannot be a suitable negative electrode material for sodium-ion batteries. People have also tried various methods to improve the sodium storage performance of graphite, but the results are not satisfactory so far. The first method is to expand the spacing of graphite layers to improve its sodium storage performance. It is found that the expanded graphite with a layer spacing of 0.43nm has a specific capacity of 184mAh/g after 2000 cycles at a rate of 5C, and a capacity retention rate of 73.92%. However, it is found from the X-ray diffraction spectrum that the ordered structure in the expanded graphite is destroyed, which is essentially the amorphization of the expanded graphite. It can make more Na+ reversibly deintercalated in graphite, but this reduced graphite oxide still has the problem of low ICE (mainly due to the unavoidable electrolyte decomposition and the irreversible relationship between Na+ and oxygen-containing functional groups on the reduced graphite oxide sheet. reaction leads to the formation of a thick SEI film), while the storage mechanism of Na+ in reduced graphite oxide remains unclear. Another approach is to improve the sodium storage performance of graphite by adjusting the electrolyte. In the experiment, it was found that the stable sodium-graphite intercalation compound could not be formed in the carbonate electrolyte, which limited the sodium storage performance of graphite. In the ether-based electrolyte, the solvated sodium ions can form Na-solvent through co-intercalation. Molecule-graphite ternary intercalation compounds are used to indirectly store sodium, but the problems of low specific capacity, high sodium storage potential, poor oxidation resistance and stability of ether-based solvents and easy reaction with positive electrodes are still the main problems of graphite as the negative electrode material of sodium-ion batteries. Difficult to overcome in practical applications. Another study shows that K ions with a larger radius than Na ions can be intercalated in graphite, and the reversible specific capacity can reach 270mAh/g, and the theoretical calculation results show that alkali metals (Li, Na, K, Rb, Cs) Na is the only intercalation compound formed with graphite, reflecting that the interlayer spacing of graphite is too small not because sodium ions cannot intercalate in graphite, but because sodium and graphite cannot form stable intercalation compounds, only Na and The formation energies of graphite-forming compounds are positive, while those of other alkali metals are negative, indicating that Na-graphite compounds are thermodynamically unstable, which is far from enough to support their commercial application as anode materials for Na-ion batteries.
Since amorphous carbon has a larger layer spacing and a disordered microcrystalline structure, it is more conducive to the intercalation and extraction of sodium ions, and has also been concerned by researchers and used in practice. As far as soft carbon is concerned, although it has a similar structure to graphite, it has a lower degree of order. Compared with graphite, it is more conducive to intercalation and storage of sodium, and can increase the specific capacity at low current density. The specific surface area and surface defects of soft carbon are low, which can reduce the consumption of ester electrolyte and help to improve ICE. From the perspective of commercialization, the soft carbon precursor is fired with cheaper anthracite, which has low price, high carbonization yield, good safety and certain electrochemical performance, and has good commercialization potential. From the perspective of application scenarios, the unmodified capacity is 200-220mAh/g, and the charging and discharging area is dominated by slopes, which is suitable for high-power scenarios. As far as hard carbon is concerned, compared with the long-range ordered layered structure of graphite, its structure at the molecular level is more complex. The picture shows the active site of hard carbon sodium storage, and it can be seen that its relatively disordered structure is more conducive to its sodium storage. The unique structure of hard carbon determines that it has various types of reversible sodium storage sites, including: sodium storage through intercalation reactions, sodium storage by forming atomic clusters in closed pores, and capacitive adsorption of sodium on the surface in contact with the electrolyte. Sodium is stored pseudocapacitively at defect-associated sites on the inner surface. The hard carbon charging and discharging area has a slope section and a platform section, and the general specific capacity can reach 300-350mAh/g. After optimization and modification, it can reach 400mAh/g, which will exceed the theoretical specific capacity of lithium battery graphite (372mAh/g).
To sum up, graphite, as an important negative electrode material for lithium-ion batteries, is quite limited in sodium-ion batteries due to the small interlayer spacing and the inability to form thermally stable intercalation compounds with graphite, although it can be achieved by expanding the layer The gap adopts expanded graphite and adjusts the electrolyte to improve this problem, but there are still problems such as low ICE and poor stability of the electrolyte. In contrast to amorphous carbon, the low degree of order of soft carbon is more conducive to sodium storage, and it also has a cheaper precursor cost. The complex molecular level structure of hard carbon has created its various types of sodium storage active sites, and optimized modification In the end, it can exceed the theoretical specific capacity of lithium battery graphite, and has strong commercialization potential. Therefore, relatively speaking, it is more appropriate to choose amorphous carbon, especially hard carbon, as the sodium ion carbon negative electrode material.
1.3 Potential competitors of hard carbon
1.3.1. Silicon-based anode materials
The advantages of silicon-based anode materials are relatively high theoretical capacity (Li4.4Si, 4200mAh/g); natural abundance (silicon is an abundant element on earth); and suitable electrochemical potential (0.4V vs Li/ Li+) - Compared with hard carbon, it is not easy to form "lithium dendrites". Of course, its disadvantages are also obvious: the inevitable volume change of the silicon material will cause the structure of the silicon-based electrode to crack or pulverize, which will lead to the uncontrollable growth of the SEI film; the conductivity itself is also poor.
1.3.2. Lithium titanate anode material
Lithium titanate negative electrode material is also a possible negative electrode material for batteries in the future. Its advantages include: simple preparation method, high charge and discharge platform, stable cycle, high Coulombic efficiency; "zero strain" material, the volume of the crystal in the reaction cycle remains stable. (Effectively solve the electrode material shedding phenomenon caused by volume change); the working voltage is stable, and lithium ions will not precipitate lithium dendrites on the electrode; the electrode voltage platform is stable. Disadvantages also exist: low conductivity and lithium ion diffusion coefficient, severe electrode polarization at high current densities, resulting in a sharp decrease in the capacitance of the electrode, and the formation of an SEI film that causes adverse reactions between the electrode and the electrolyte for a long time. At present, the improvement scheme of lithium titanate negative electrode material: (1) Nanoization: effectively reduce the propagation of lithium and electrons in the electrode, and increase the charge migration on the electrode surface; (2) Surface coating (metal element Or carbon material): improve The electrical conductivity of the negative electrode material; block the direct contact between the lithium titanate material and the electrolyte to produce side reactions; (3) Compound modification: introduce materials with good electrical conductivity; (4) Doping modification: apply conductive properties to lithium titanate ions Substances (currently the most used are various types of carbon and highly conductive metal elements).
1.3.3. Tin-based anode materials
Tin-based anode materials have also attracted widespread attention from scholars and entrepreneurs. Its advantages lie in: rich resources (as of 2019, the proven reserves of tin mines in China are 2.16 million tons, accounting for 39.27% of the world, ranking first in the world); high theoretical capacity (Li22Sn5 produces a total theoretical capacity of about 994mAh/g, Na15Sn4 Produces a theoretical capacity of 847mAh/g); the lithium intercalation potential is higher than the lithium precipitation potential, and lithium deposition can be avoided at high rates; the bulk density is large (75.46mol/L, close to the bulk density of lithium 73.36mol/L). The disadvantage is that the volume expansion rate of Sn during the cycle reaches 259% (Li-ion battery) and 423% (Na-ion battery), which seriously affects the cycle performance. Current improvement measures: (1) Reduce the particle size of Sn to nanoscale: there may be uneven particle size and agglomeration, and the advantages of high capacity of Sn cannot be demonstrated; (2) Tin-based alloy material: lithium-ion battery negative electrode The material has entered a stable development stage - most of the tin-based alloys and heterogeneous components are only physically composited, and the weak interfacial force is not conducive to the recombination effect; the anode materials for sodium-ion batteries are being explored; (3) tin-based/ Carbon composite material: Sn alloy particles wrapped in an elastic carbon shell can stably complete the process of lithium intercalation and desorption.
2. Rediscovering amorphous carbon: what determines its performance?
2.1, hard carbon vs soft carbon
According to the difficulty of graphitization, amorphous carbon materials can be divided into hard carbon and soft carbon. Soft carbon usually refers to a carbon material that can be graphitized after high temperature treatment (above 2800 ° C), and the disordered structure is easily eliminated, also known as graphitizable carbon. Hard carbon usually refers to carbon that is difficult to completely graphitize after high temperature treatment (above 2800 ° C), and its disordered structure is difficult to eliminate at high temperature, also known as non-graphitizable carbon. Under medium and low temperature (1000-1600°C) treatment, there is no clear boundary between soft carbon and hard carbon in structure, which can be collectively referred to as amorphous carbon.
Although soft carbon materials have a higher capacity value, their fast decay rate has caused obstacles to practical applications; hard carbon materials are easier to prepare and have a higher cycle life, and have been used in some practical applications. Compared with soft carbon, hard carbon has more disordered structure, higher defect concentration, higher heteroatom content and larger distance between graphitic layers, and more closed pore structure. This is beneficial to provide more storage points and diffusion pathways for Na+ ions. However, the economy of hard carbon is slightly worse than that of soft carbon. Among the sodium-ion batteries, hard carbon is the mainstream of current applications due to its advantages. In addition, the characteristics of low cost, sustainability, and simpler preparation also provide more possibilities for the commercialization of hard carbon materials.
2.2. Precursor
Soft and hard carbons mainly depend on the properties of the precursors. In the carbonization process, the precursor can appear in a fusion state in a wide temperature range, which is a necessary condition for the final carbon (coke) to be graphitized. This fused state allows the rearrangement of the carbon layers to form a long-range ordered layered structure, and the gas generated by thermal decomposition is easy to escape, while the carbon content and density of the residue are increased. Amorphous carbon is usually produced by the pyrolysis of organic precursors at 500–1500 °C. Whether the final product after pyrolysis is hard carbon or soft carbon mainly depends on the properties of the precursor.
Precursors are mainly divided into biomass-based, polymer-based, resin-based and coal-based carbon materials. Biomass precursors mainly refer to the roots, stems and leaves of plants (for example: banana peels, peat moss, peanut shells, leaves, apple peels, grapefruit peels, poplar and cotton, etc.). Polymers usually refer to carbohydrate precursors, mainly including glucose, sucrose, starch, cellulose and lignin, which are chemical products extracted from biomass. Resin precursors mainly include phenolic resin, polyaniline and polyacrylonitrile. The precursors used to produce hard carbon are mainly biomass, resin and polymer precursors. The precursors for preparing soft carbon materials mainly include petrochemical raw materials and their downstream products, such as coal, pitch, petroleum coke, etc., but direct carbonization Soft carbon materials exhibit low reversible capacity in Na-ion batteries.
Amorphous carbon has the characteristics of excellent reversible capacity and cycle performance, and it is expected to be commercialized after controlling the cost. Hard carbon materials have high gram capacity, but high cost; soft carbon materials have low gram capacity, but have cost-effective advantages. The core of the anode material for sodium ion battery is how to reduce its cost.
The core technical route of hard carbon preparation includes raw material selection and pretreatment, cross-linking and curing, carbonization and purification, etc. Different types of precursors also have technological differences in the preparation process of hard carbon anode materials. The temperature control of the intermediate steps, gas atmosphere, heating time, etc. affect the pore size, purity, oxygen content, specific surface area, etc. of the negative electrode material. It also indirectly affects the initial efficiency, energy density, safety and other factors of the battery.
The molecular structure of the organic polymer precursor is relatively simple and controllable, and the relevant molecular structure can be designed according to the needs. Therefore, it is an excellent precursor for the preparation of carbon materials and has attracted widespread attention. Organic macromolecular polymers are prepared from organic small molecular monomers through catalyst-catalyzed polymerization. Because of the advantages of obtaining a hard carbon structure with regular morphology and simple synthesis process, it is of great significance for the large-scale production and application of hard carbon materials in the future. high research value. Phenolic resin material (RF) is a well-studied organic polymer at present. It has received extensive attention because of its high residual carbon rate and excellent structural stability after forming hard carbon. It was found that the time required for polymerization depends on the solvent used, while the yield depends on the degree of crosslinking, which can be controlled by adjusting the temperature of thermal polymerization. The structure of hard carbon mainly changes with the carbonization temperature, and as the temperature increases, the interlayer spacing and disorder decrease, which affects the sodium storage performance of the material. And it is beneficial to improve the electrochemical performance by regulating the formation of hard carbon pore structure. Foreign Kamiyama team found that as the heat treatment temperature increased, the interlayer distance decreased, the specific surface area decreased, and the internal pores increased. The unique macroporous phenolic resin increases the total volume of the internal pores, thereby increasing the sodium ion holding capacity.
Biomass precursors are rich in variety, sustainable use and low cost. Usually contains a lot of C, and contains some O, H, and even some other heteroatoms, such as N, S, P, etc. Biomass is a good choice as a renewable and sustainable precursor for the production of low-cost and high-performance hard carbon anode materials. The methods to convert biomass to hard carbon are simple, such as direct carbonization, hydrothermal carbonization (HTC), physical or chemical activation, etc. Biomass such as banana peel, peat moss, rice husk, cotton, glucose, protein, and cellulose nanocrystals have been used as anode materials for Na-ion batteries, showing promising electrochemical performance. The third-generation high-end customized biomass hard carbon anode material deployed by JiaNa Energy has a specific capacity of about 400mAh/g. This indicates that biomass has great potential in promoting the development of anodes for Na-ion batteries. However, on the other hand, biomass from nature usually contains some impurities that need to be removed before it can be applied to Na-ion batteries. Most of the reported biomass-derived hard carbon anodes can deliver reversible capacities up to 300mAh/g with ICE (coulombs in the first week) below 85%, which still cannot compete with graphite anodes in commercial lithium batteries. Although biomass is sustainable and abundant, the overall cost is still higher than that of graphite and soft carbon.
As a low-cost petrochemical by-product, bitumen is currently widely used due to its low cost and high carbon content. However, the asphalt base can easily form an ordered structure during pyrolysis, so its storage capacity is very low, less than 100mAh/g. At present, the Chinese Academy of Sciences has improved the sodium storage capacity to 300mAh/g by compounding pitch as a soft carbon precursor and resin as a hard carbon precursor for modification.
Anthracite-based precursors are cost-effective and have broad market application prospects. The Institute of Physics of the Chinese Academy of Sciences used anthracite as a precursor to obtain a carbon anode material with excellent sodium storage properties through simple crushing and one-step carbonization. The soft carbon material obtained by cracking anthracite still has a high degree of disorder below 1600°C, the carbon production rate is as high as 90%, the sodium storage capacity reaches 220mAh/g, the cycle stability is excellent, and the performance is better than the soft carbon material from asphalt. carbon material.
Zhongke Haina also investigated the carbon source precursors and found that the cost of anthracite is low. Using anthracite to prepare amorphous carbon negative electrode materials will help greatly reduce battery costs. Through experiments, anthracite-based sodium-ion battery negative electrodes were finally developed. Material. Using anthracite as the precursor can reach 150-300Ah/yuan, which has a significant cost-effective advantage over other precursors.
3. Focus on hard carbon: demand geometry?
3.1. Lithium battery hard carbon demand forecast
The application of hard carbon in the field of lithium-ion batteries At present, most domestic enterprises that deploy hard carbon apply it to lithium-ion batteries, and have achieved relatively rich results and practices. In the selection of negative electrode materials for lithium-ion batteries, graphite has become the main raw material. The structural defects of graphite negative electrode limit its cycle stability and charge-discharge efficiency as a lithium-ion battery negative electrode material, while hard carbon has isotropic structural characteristics, larger interlayer spacing, and fast lithium ion diffusion during charge and discharge. With good rate performance, hard carbon has a better application in the field of lithium-ion batteries.
As mentioned above, hard carbon is an important anode material for sodium-ion batteries. Its excellent specific capacity and low price are its important advantages in many anode materials. The lithium-ion battery, which is also a "rocking chair battery", can actually use hard carbon as its negative electrode material because of its high similarity to the principle of sodium batteries. In the negative electrode materials of lithium-ion batteries, natural graphite and artificial graphite have always occupied a large proportion. However, as people's requirements for battery energy density gradually increase in the future, the theoretical upper limit of graphite specific capacity of 372mah/g will no longer meet the demand. At that time, silicon anode materials and hard carbon materials that can also achieve higher specific capacity will have a greater performance stage. At present, silicon anode materials have not been fully applied in batteries, but hard carbon materials have already occupied a certain proportion in lithium battery anode materials.
In 2021, the shipment structure of my country's lithium battery anode products is still dominated by artificial graphite, accounting for 84%; natural graphite is the second largest segmented anode product, accounting for 14%; the rest of the anode materials are 2% . In other parts, hard carbon and soft carbon materials are the main parts. According to Juda Lithium Battery data, soft carbon and hard carbon materials accounted for 1.7% of the global lithium battery anode material shipments in 2015. In recent years, the application of hard carbon materials in lithium batteries has also made some industrial progress. Therefore, we predict that in the next few years, hard carbon materials will be an application material for lithium battery negative electrodes, accounting for about 2%. The future shipments of lithium batteries are showing a trend of rising all the way. With the continuous increase in the penetration rate of global new energy vehicles and the gradual advancement of the dual carbon targets, the demand for power batteries and energy storage batteries will continue to grow at a relatively high rate, and before 2030, other battery systems are still difficult to industrialize on a large scale Development, lithium-ion batteries will remain the mainstream technology route. EVTank predicts that the compound annual growth rate of global lithium-ion battery shipments will reach 25.6% before 2030. Global lithium battery shipments will reach 562.4Gwh in 2021. Based on the compound growth rate of this year, it is estimated that global lithium battery shipments will reach about 1554.6Gwh in 2025.
Since the proportion of hard carbon in lithium battery anode materials is not high, lithium batteries will have less pull on hard carbon materials. According to the hard carbon 300mah/g capacity, 3.2V voltage platform calculation, 1GWh lithium battery consumes about 1125 tons of hard carbon, and we expect that by 2025, about 35,000 tons of hard carbon materials will be used for the production of lithium battery anode materials.
3.2 Forecast of demand for sodium battery hard carbon
The characteristics of hard carbon and its application scenarios in sodium-ion batteries: Recently, "Towards enhanced sodium storage of hard carbon anode materials: regulating the oxygen content of precursors through low-temperature hydrogen reduction reaction" (Towards enhanced sodium storage of hard carbon anodes: Regulating the oxygen content in precursor by low-temperature hydrogen reduction) was published in the journal Energy Storage Materials. When the research team tested the electrochemical performance of hard carbon materials, they found that when they were used as negative electrode materials for sodium-ion batteries, a sample showed 369.8mAh/ g high specific capacity; Southern University of Science and Technology Lu Zhouguang research group found that hard carbon has a low redox potential (0.1-1.0V). Due to the widespread use of hard carbon precursors, biomass-related precursors, hard carbon has become a green and environmentally friendly battery anode material choice. In summary, in the application field of sodium ion batteries, hard carbon has a larger interlayer distance than graphite and can form a thermodynamically stable intercalation compound with sodium. Compared with soft carbon, it has a larger sodium storage capacity. Capacitor electrodes, sodium-based dual-ion battery electrodes, and other fields related to sodium-ion batteries have good application scenarios, and have been researched and put into application by companies such as Betray and Shanshan New Energy.
In the "White Paper on the Development of China's Sodium-Ion Battery Industry (2022)" jointly released by EVTank and Evie Economic Research Institute, the energy density of sodium-ion batteries, lithium iron phosphate batteries, ternary batteries and lead-acid batteries is comprehensively compared and analyzed. , cycle life, average voltage, safety, rate performance, fast charging performance, high and low temperature performance, etc., it is believed that sodium ion batteries have relatively good application scenarios such as electric two-wheel vehicles, low-speed electric vehicles, energy storage, start-stop, etc. Good prospect. EVtank believes that in theory, the market space of sodium-ion batteries can reach 369.5GWh in 2026 under the condition of 100% penetration, and its theoretical market size may reach 150 billion yuan.
Assuming that the replacement ratios of sodium batteries from 2023 to 2025 are 5%, 15%, and 25%, respectively, the corresponding installed capacity of sodium batteries is 9GWh, 33.7GWh, and 72.5GWh. According to the hard carbon 300mah/g capacity, 3.4V voltage platform calculation, 1GWh sodium battery consumes about 1075 tons of hard carbon, we predict that the demand for hard carbon corresponding to sodium batteries in 2023-2025 will be 9,700 tons, 36,200 tons, and 77,900 tons .
Summarizing the two parts of hard carbon demand, we estimate that the total hard carbon demand in 2021 will be about 12,700 tons, and the total hard carbon demand in 2025 is expected to increase significantly to about 112,900 tons, with a compound annual growth rate of 72.8%.
4. Looking forward to the future: the process of industrialization is just around the corner
4.1. Domestic hard carbon enterprises
Judging from the deployment of hard carbon by domestic companies, many companies choose to use hard carbon in the field of lithium-ion batteries to enhance performance. According to the company's invention patent information, lithium-ion batteries using hard carbon negative electrode materials can achieve a reversible capacity of 450mAh. /g or more, and the first effect can exceed 50%. Taking Xiangfeng Huawei as an example, the initial discharge capacity of sodium batteries can reach 230-260mAh/g, and the first efficiency can reach about 90%. At the same time, more companies are deploying hard carbon in battery anode materials.
4.2 Pain points of hard carbon industrialization
4.2.1 Pain points and improvement measures of industrialization of hard carbon in sodium-ion batteries
(1) The study of hard carbon structure needs to be deepened
A well-performing hard carbon needs to have a low external surface area to minimize the first-cycle irreversible charge loss associated with SEI formation and a high concentration of internal micropores to maximize specific charge storage. Among them, the research method of gas adsorption intelligently detects open micropores, but is relatively insensitive to internal micropores. Corresponding methods such as HR-TEM, X-ray diffraction correlation measurement, PDF analysis of PXRD, and small-angle X-ray scattering are suitable for hard carbon The research on the structure is still uncertain and difficult, and it needs to be further improved in terms of hard carbon measurement-related accessories and angle correction. Industrial application of sodium ion batteries.
(2) The hard carbon process is complicated, and there are problems such as precursor defects
The structure of hard carbon materials depends on the state of the precursor and the corresponding carbonization process. First of all, although the biomass precursor has the advantage of low cost, it contains more heteroatoms with uneven distribution, which seriously affects the industrialization process of large-scale preparation of high-quality hard carbon. Secondly, the synthesis process of hard carbon is relatively complicated, which greatly increases the manufacturing cost of hard carbon and affects large-scale production. The hard carbon production process mainly starts with pretreatment of raw materials, followed by cross-linking and solidification, medium-temperature carbonization, deep carbonization, surface modification, and high-temperature carbonization, and finally forms hard carbon. Again, hard carbon has a lower carbon yield than soft carbon. In addition, various precursors of hard carbon have some advantages and disadvantages. Although asphalt materials have a high carbon residue rate and a wide range of raw material sources, their composition is complex, and the quality is prone to unevenness between batches during the preparation process. At the same time, the asphalt needs to improve the tail gas treatment process during the treatment process; Although biomass is a source of high-quality carbon, it is a macromolecule, and it is difficult to design materials from the molecular level. At the same time, biomass materials are restricted by seasons and environments, and their sources are unstable. The production cost of organic polymers is relatively high, and there are still some difficulties in large-scale preparation. In summary, the structure of electrode materials for sodium batteries requires consistency. Due to the presence of heteroatoms in hard carbon biomass precursors, low carbon production rate and complicated hard carbon process, the manufacturing cost of hard carbon as a sodium battery material remains high, resulting in There are limitations to the industrialization of hard carbon in the field of sodium-ion batteries.
Due to the high production cost of hard carbon precursors, one possible reasonable solution is to mix suitable materials with hard carbon precursors to jointly prepare negative electrode materials for sodium batteries. The Institute of Physics of the Chinese Academy of Sciences invented a carbon material for negative electrodes of sodium-ion batteries and its preparation method, as well as negative electrodes of sodium-ion batteries and sodium-ion batteries in the patent. It uses cheap coal as the main raw material and mixes it with hard carbon precursors. Amorphous carbon materials, taking the experimental results of anthracite, lignin (1:0.1) and carbon coating as an example, the material has a specific capacity of 229mAh/g, the Coulombic efficiency of the first week is 88%, and the residual carbon of anthracite The rate can reach 90%. As an amorphous carbon material with simple preparation, readily available raw materials, low cost and high carbon production rate, it is more suitable for the negative electrode material of sodium-ion secondary batteries.
(3) Low-voltage platform application scenarios account for a relatively low proportion, and sodium deposition affects performance
In recent years, researchers have studied different Na+ storage mechanisms. Based on the phenomena consistent with their respective experiments, the sodium storage mechanisms are divided into "intercalation-filling" mechanisms, "adsorption-intercalation" mechanisms, and "adsorption-filling" mechanisms. "Adsorption-intercalation-filling" mechanism. The research shows that through the analysis of diffusion coefficient, ex-situ XRD and sodium deposition experiment results, the storage mechanism in the slope area belongs to the adsorption of Na+, while the storage mechanism in the low voltage plateau area is due to the intercalation of Na+ and a small amount of Na+ in the Adsorption on the pore surface. Although the distribution of sodium storage mechanism in different voltage regions is controversial, the discharge process can be generally divided into two parts, that is, the slope part above 0.1V and the plateau part below 0.1V; three corresponding interaction modes of sodium and hard carbon , that is, adsorption of surface active sites, intercalation of graphite layers and pore filling. Further studies have found that hard carbon sodium storage has an obvious platform area, that is, the sodium storage potential is below 0.1V, and the hard carbon anode with a long sodium storage platform can improve the average working voltage and energy density of the battery. However, the platform potential below 0.1V is close to the potential of sodium deposition. Britton et al. used nuclear magnetic resonance spectroscopy to discover the development and changes of metal Na and metalloid Na during charging cycles, and it is related to the intercalation of sodium in hard carbon electrodes. Whether the hard carbon surface will further form sodium dendrites due to the deposition of metallic sodium, and whether the sodium dendrites will pierce the separator and cause battery safety issues, all of these need further detection and research, and the application of hard carbon in the field of sodium batteries is subject to battery safety. Aspects of influence and constraints.
(4) Hard carbon has low first-week coulombic efficiency and cycle life
In the current reports and researches, the Coulombic efficiency of the hard carbon negative electrode is usually low in the first week, usually 50%-80% in the ester-based electrolyte, and a few exceed 80%; usually 80%-90% in the ether-based electrolyte. %, up to 93%; in contrast, the first-week coulombic efficiency of graphite anodes for commercial lithium-ion batteries usually exceeds 95%, and the application of hard carbon in the field of sodium-ion batteries is limited. In addition, the cycle stability of the hard carbon negative electrode also needs to be improved, and the different experiments in the table also illustrate this problem. Among them, Hu et al. proved that the capacity loss during the charge-discharge cycle of hard carbon is not due to irreversible damage to the microstructure; Younesi et al. found that most of the SEI film formed on the surface of hard carbon in sodium ions will dissolve, and its stability is better than that of lithium-ion batteries. The SEI film formed in is worse, and the passivation of the hard carbon electrode surface is not sufficient.
In the process of pre-sodiumization, how to avoid cumbersome and time-consuming problems, and how to suppress the decomposition side reaction of the electrolyte on the carbon surface as much as possible is one of the research focuses of the pre-sodiumization strategy. Zheng et al. chose reduced graphene oxide (rGO) with high rate and low first effect as the model carbon material, sodium naphthalene (Na-Nt) as the pre-sodiumizing agent, and achieved rapid pre-sodiumizing within 10s, increasing the ICE from 78% to 96.8%. In this process, a stable artificial SEI is formed, which effectively avoids the continuous decomposition of the electrolyte and the irreversible consumption of Na+. In addition, it also exhibits a sodium storage capacity of 200mAh/g at a high current of 5A/g and a sodium storage capacity of 0.5A/g The capacity retention rate is 68.4% after 1000 cycles under the current density. This study has implications for the presodiumization of hard carbon materials. Depending on the storage form of hard carbon, the adsorption and reaction can be induced by: expanding the interlayer spacing of graphite to allow large-radius Na+ insertion; optimizing the pore network to facilitate Na+ transport or storage; introducing heteroatoms or defects into the carbon lattice. For improving its electrochemical performance, the platform capacity can be increased by means of heteroatom doping and high heating rate, etc., by using S, P, B, N, F doping or co-doping carbon and bulk five-membered rings, Intrinsic defects such as seven-membered rings are used to improve the slope capacity and rate performance of hard carbon materials, and low heating rate, coating, pre-sodiumization and other means are used to reduce defects and improve the first effect of materials.
4.2.2 Pain points and measures of industrialization of hard carbon in lithium-ion batteries
(1) The first discharge efficiency is low
Compared with graphite, hard carbon itself has a larger specific surface area. The larger the contact area of the corresponding negative electrode interface with the electrolyte, the greater the possibility of chemical reactions at the interface, and more SEI films will be formed, and the lithium ions consumed will be more If there is more, the lithium used for migration will be reduced. At the same time, Buiel et al. found that lithium also participates in the impurity reaction adsorbed in the hard carbon nanopores and consumes lithium, thereby reducing the first discharge efficiency of hard carbon materials.
Studies have shown that when artificial graphite is doped with 10% hard carbon, the battery will produce the least side reactions during formation and consume less lithium ions, which will not affect the battery capacity. At the same time, due to the structural advantages of hard carbon itself , the low-temperature charge-discharge performance and the normal-temperature high-temperature cycle performance have been greatly improved.
(2) The hard carbon process is complicated, and there are problems such as precursor defects
The carbon yield of hard carbon precursors is low and the preparation process is complicated, which brings about high preparation costs. Related content has been mentioned in the field of sodium-ion batteries and will not be repeated here.
(3) Hard carbon has a large irreversible capacity
Studies using sucrose as the precursor material show that there are generally two mechanisms for the irreversible capacity formation of hard carbon: Lithium reacts with the electrolyte to form an SEI film that consumes lithium; Lithium reacts with surface functional groups or absorbing molecules exposed to the air after pyrolysis, The reaction between lithium and electrolyte contributes about 50mAh/g to the irreversible capacity, while the second part is aimed at hard carbon, which can contribute more than 150mAh/g to the irreversible capacity, which is an important factor restricting the industrialization of hard carbon. Taking the sucrose precursor as an example, the chemical vapor deposition of ethylene gas above 700 degrees can be used to reduce the irreversible capacity contribution above 150mAh/g to less than 70mAh/g.
(4) Voltage lag
The electrode potential of hard carbon materials is extremely high compared to graphite, and the delithiation potential is greater than the lithium intercalation potential, which is easy to form voltage hysteresis. Buiel E et al. believe that the voltage hysteresis during the charging and discharging process of hard carbon is caused by less than 0.5% hydrogen remaining in pyrolysis. The voltage hysteresis is not conducive to the maintenance of charging of lithium-ion batteries and affects the practical process of hard carbon materials. Mixing hard carbon materials with graphite materials can improve the problem of voltage hysteresis. Nei P. Dasgupta conducted continuous-scale electrochemical simulation analysis. The mixed material will be preferentially lithiated in the early stage of fast charging, and the good rate characteristics of hard carbon reduce the current on graphite particles to avoid lithium precipitation. With the increase of , lithium ions will be gradually intercalated into graphite, thereby improving the available capacity of the hybrid electrode and increasing the energy density.
(This article is for reference only and does not represent any investment advice from us. For relevant information, please refer to the original report.)

