At present, the ternary cathode materials NCM111, NCM523 and NCM622 for lithium-ion batteries have been put into mass production. From the perspective of cathode materials, the increase of nickel content will lead to the intensification of Li/Ni mixing in the ternary material and shorten the cycle life.
More seriously, the increase of nickel content will lead to a significant increase in the residual alkaline impurities between particles, which will lead to serious gas production during the charging and discharging process, resulting in bulging deformation of the battery, shortened cycle and shelf life, and potential safety hazards. The residual alkaline impurities have become the key to restrict the application of high-nickel ternary materials in high-energy-density power batteries for electric vehicles.
In addition, in recent years, various methods such as anionic and cationic doping or coating have been adopted to stabilize the bulk phase structure of ternary materials and achieve the effect of improving cycling and storage performance. These methods are difficult to solve the problem of high residual alkali impurities in high nickel materials. To this end, the author of this paper studied the residual alkali impurities under different sintering temperatures and lithium/metal ratios during the preparation of NCM811 materials by high-temperature solid-phase method, and verified the alkali reduction effect of various post-treatment systems.
1. Experiment
1. Synthesis of NCM811 material
The NCM811 material precursor Ni0.8Co0.1Mn0.1(OH)2 and LiOH are mixed according to the set stoichiometric ratio. In a high-speed mixer, the raw materials were mixed at a speed of 700r/min for 0.5h. The mixture was placed in a corundum crucible, placed in a box-type atmosphere sintering furnace, sintered in an oxygen atmosphere and at a specific temperature for 12 hours, and naturally cooled to room temperature in an oxygen environment to obtain the NCM811 sample. Three test points were selected in different lithium ratios and temperature regions. The n(Li):n(Ni+Co+Mn) and sintering temperatures corresponding to each sample are shown in Table 1.
2. XRD and SEM analysis of samples
The structure of the sample was analyzed by powder X-ray diffractometer, CuKα, wavelength 0.15406nm, tube pressure 40kV, tube flow 40mA, scanning speed 2 (°)/min, step size 0.02°. The surface topography of the samples was analyzed by field emission scanning electron microscopy.
3. Analysis and treatment of residual alkaline impurities in samples
NCM811 (made in Japan), NCM622 (made in Hunan), NCM523 (made in Zhejiang) and NCM111 (made in Fujian) were selected as comparative samples for the analysis of basic impurities. Place 5g of powder sample in 95g of deionized aqueous solution, stir for 5min and then suction filter. Use a potentiometric titrator to calculate the contents of Li2CO3 and LiOH in the solution according to the equivalent point values V1 and V2, and use the contents as impurities to represent the composition.
According to the content of Li2CO3 and LiOH in the measured sample 2, the above-mentioned basic impurities of 20%, 50% and 80% of the theoretical value were consumed as the end points, and the alkali-reducing substance ammonium dihydrogen phosphate was calculated and added. After fully reacting and evaporating to dryness with constant stirring, backfired in an oxygen environment of 700° C. for 5 h to obtain sample 2-P2, sample 2-P5, and sample 2-P8. In addition, with the solid-liquid mass ratio of 1:4, the comparative sample 2-H2O was prepared by rinsing with pure water, and the backburning conditions were the same.
4. Positive pole piece fabrication and simulated battery assembly
The NCM811 sample, polyvinylidene fluoride, and acetylene black were mixed in a mass ratio of 92:5:3, and after grinding uniformly, coated on a 0.1 mm thick aluminum foil, and punched into a circular positive plate with a diameter of about 14 mm, which contained about 10 mg The positive electrode material was finally vacuum-dried at 120 °C for 12 h. A CR2032 button cell was assembled in an argon-protected glove box with lithium metal sheet as the negative electrode, Celgard 2325 film as the diaphragm, and 1 mol/L LiPF6/EC+DMC (mass ratio 1:1) as the electrolyte.
5. Electrochemical performance test
The charge-discharge test was carried out at 22°C with CT4008 battery performance test system. Rate performance test: at 3.00-4.30V, cycle at 0.10C, 0.20C, 0.50C, 1.00C, 2.00C and 5.00C in turn, and compare the discharge specific capacity with the value at 0.01C. Cycle performance test: first charge to 4.3V with 1.00C constant current, turn to constant voltage to charge until the current is 0.01C; then discharge with 1.00C constant current to 3.0V.
2. Results and discussion
1. XRD analysis of sintered material
Figure 1 is the XRD pattern of the prepared NCM811 material. As can be seen from Figure 1, NCM811 prepared under different temperature and lithium ratio conditions did not appear impurity peaks, and each sample was of α-NaFeO2 structure. The degree of splitting of the (006)/(102) and (108)/(110) crystal planes can usually be used to measure the degree of order of the layered two-dimensional structure.
2. Comparison of residual alkaline impurities
Generally speaking, using the sintering conditions of high temperature and low lithium ratio, the residual alkaline impurities in the prepared cathode material will be lower than that of the samples prepared under the condition of low temperature and high lithium ratio. High alkaline impurities will lead to a rapid increase in the viscosity of the coating slurry during the use of the material, and even a "jelly" phenomenon; in addition, it will also lead to a series of problems such as the reduction of the maximum compaction of the pole piece and the bulging during the cycle. The comparison of the basic impurity content of the prepared NCM811 sample and the purchased sample is shown in Table 2.
It can be seen from Table 2 that although the process conditions of high sintering temperature and low lithium ratio addition were selected for sample 3, the residual mass fraction of basic impurity lithium carbonate in the prepared NCM811 material was still 1.22%, lithium hydroxide was 0.69%, high For purchased ternary material products.
From the trend of changes in the content of basic impurities in the commercialized NCM111, NCM523 and NCM622 materials, it can be seen that with the increase of Ni content, the residual content of basic impurities also increases, and the degree of increase is higher than the linear increase. This is due to the inherent characteristics of nickel-containing ternary materials. The optimization of the sintering process can reduce the residual amount of basic impurities, but for high nickel ternary materials such as NCM811, other means must be used to reduce the content of basic impurities.
3. Alkali reduction technology and effect
The reaction in liquid phase environment is a direct solution to realize the separation or transformation of basic impurities, among which, phosphate coating is an effective modification method. The idea can be transformed into: NCM811 is used as the matrix and ammonium dihydrogen phosphate is used as the modified substance for treatment, and through secondary sintering at 700 ℃, an attempt is made to form a stable and fast ion conductor layer on the surface of the NCM811 material that can protect the surface of the material. The purpose is to consume residual alkaline impurities and improve material performance.
In addition, the scheme for the separation and transformation of basic impurities in the study is: the NCM811 material is treated with pure water rinsing, and the water-soluble characteristics of basic impurities are used to realize the separation of surface basic impurities from the system (sample 2-H2O ). The basic impurity contents of the samples obtained by different alkali reduction processes are shown in Table 3.
It can be seen from Table 3 that with the increase of the amount of phosphate added, the content of LiOH and Li2CO3 remaining on the surface of NCM811 was significantly reduced, indicating that the alkaline impurities were consumed during the treatment process. Compared with Li2CO3, LiOH has a greater reduction, which may be due to:
① During the treatment process, LiOH is converted to Li2CO3; ② During the treatment process, Li in the structure is precipitated, and new basic impurities appear again during the backburning process;
The specific mechanism needs further experimental study. For the sample 2-H2O prepared by pure water rinsing, the content of basic impurities in the material is significantly reduced.
4. XRD analysis of samples before and after alkali reduction process
Figure 2 shows the XRD comparison of samples before and after treatment with different alkali reduction processes.
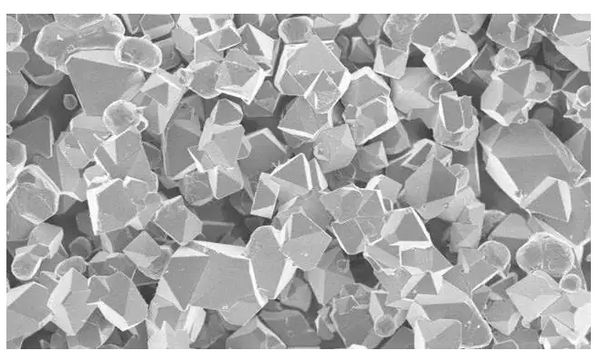
5. SEM analysis of samples before and after alkali reduction process
The SEM images of sample 2 and the sample after alkali reduction treatment are shown in FIG. 3 .
It can be seen from Figure 3 that there is an obvious dark area on the surface of sample 2, there is no fixed morphology, and it should be a weakly conductive alkaline impurity containing lithium remaining on the surface of the particles; in sample 2-P2 after treatment, no obvious At the same time, a thin coating layer is formed on the surface of the particles. With the increase of the amount of phosphate added, the clarity of the particle surface gradually decreased, and the particle boundary was gradually blurred. The sample 2-H2O after water washing has clean particle surface, clear boundary and larger particle gap. From the perspective of particle morphology, both treatment methods can realize the separation or harmless treatment of alkaline impurities.
6. Electrochemical properties of samples before and after alkali reduction process
The half-cell rate data of sample 2 and the sample after alkali reduction treatment are shown in Figure 4.
Figure 4 combined with the data in Table 3 shows that the higher the residual amount of alkaline impurities, the lower the Coulombic efficiency of the first cycle of the half-cell. The first charge-discharge efficiency of the sample after washing with water was the highest, reaching 93.0%. Comparing the rate performance of samples with different treatments, it can be seen that sample 2-P2 and sample 2-H2O show similar rate levels, which are slightly lower than that of sample 2. From Fig. 3(b), (e), it can be observed that the primary particles of sample 2-P2 and sample 2-H2O have smoother and cleaner surfaces than those before treatment. Therefore, the small decrease in rate performance should originate from the particle surface. Lithium salt species that can function as Li+ conductors are removed.
With the increase of phosphate addition, the rate performance of the samples decreased. In Figure 3, samples 2-P5 and 2-P8 are enriched with dark and flocculent substances on the surface and gaps of primary particles, indicating that the amount of phosphate added under these conditions is too high, and the formed substances have weak electronic conductivity, hindering the Li+ conduction in the bulk phase of the cathode material.
The cycle performance of sample 2 and the half-cell prepared by the sample after alkali reduction treatment is shown in Figure 5.
It can be seen from Figure 5 that the 1C specific capacity of the phosphate-treated sample is significantly lower than that of the untreated sample 2, and with the increase of the amount of phosphate added, the specific capacity decreases by about 1mAh/g (sample 2-P2) and 27mAh respectively. /g (Sample 2-P5) and 37mAh/g (Sample 2-P8), meanwhile, the capacity retention rate is also lower than that of Sample 2. Phosphate treated samples formed inert species on the surface that did not provide capacity. The worsening cycle retention of the phosphate-treated samples compared to the untreated samples proves that lithium atoms may be extracted from the structure during treatment.
In contrast, the first specific capacity of 2-H2O washed with pure water increased from 179.2 mAh/g to 181.8 mAh/g, the discharge specific capacity after 100 cycles was still about 171 mAh/g, and the capacity retention rate reached 94.1%. The increase in discharge specific capacity may be due to the fact that water washing greatly reduces the content of electrochemically inert alkaline impurities remaining on the surface of the NCM811 material. At the same time, the removal of impurities on the surface and interparticle gaps enables the NCM811 material to have enough active surface to achieve primary particles and electrolytes. full contact. The experimental results show that the removal of alkaline impurities is beneficial to improve the capacity retention rate of cathode materials.
3. Conclusion
In this paper, the author prepared a high-nickel ternary cathode material NCM811 through a high-temperature solid-phase synthesis process. The product is α-NaFeO2 structure without impurity phase. Using the sintering conditions of low lithium ratio and high sintering temperature, the residual basic impurity content of the sample is still higher than that of NCM523, NCM111 and other low nickel content ternary materials, indicating that high basic impurities are the common characteristics of high nickel ternary materials.
In a liquid phase environment, NCM811 was treated with different amounts of ammonium dihydrogen phosphate and secondary sintered. The results show that the residual alkali content on the surface of the material is reduced, and the crystal structure does not change, but electrochemically inert substances remain on the surface of the particles, resulting in a significant decrease in capacity and cycle retention. This shows that the idea of reducing residual alkaline impurities by conversion is feasible, but it is necessary to further optimize the amount of phosphate added and the temperature of backburning.
Using water washing can greatly reduce the content of alkaline impurities, and it is lower than the level of imported products. Compared with the sample before treatment, the specific capacity increased by about 1.5mAh/g, and the capacity retention rate after 100 cycles also increased from 90.8% before treatment to 94.1%.
The above results show that water washing is a convenient and effective method to control the content of alkaline impurities in high-nickel ternary materials and improve the properties of materials. As the basic process of controlling residual alkali in high nickel ternary materials, water washing can exert the performance of the material itself. The follow-up needs to focus on how to combine water washing and alkali reduction with coating, so as to directly convert the residual basic impurities of alkaline impurities into the coating layer that can realize Li+ conduction on the surface of the primary particles of the positive electrode material, so as to further improve the utilization rate of lithium resources and at the same time. Improve the electrochemical performance of cathode material products such as cycle and thermal stability.

