Electrochemical flow cell is generally called flow redox cell or redox flow cell is a new type of large-scale electrochemical energy storage device. The vanadium salt solution is used for both positive and negative electrodes, which is called all-vanadium redox flow battery, or vanadium battery for short. The open circuit voltage of the battery can reach 1.5 V at 100%.
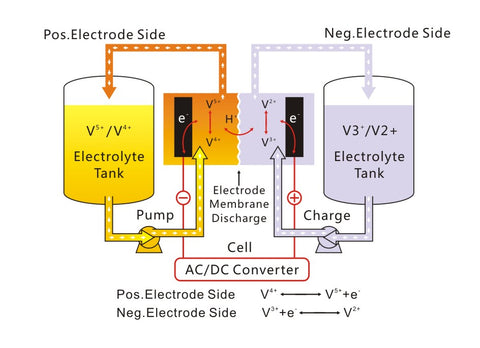
Working principle
The liquid flow battery is a new type of storage battery. The flow battery is a high-performance storage battery that uses the positive and negative electrolytes to separate and circulate separately. With the characteristics of high capacity, wide application field (environment), and long cycle life, it is a new energy product at present. Redox flow battery is a new type of large-capacity electrochemical energy storage device that is being actively developed. It is different from batteries that usually use solid material electrodes or gas electrodes. Its active substance is a flowing electrolyte solution. Its most notable features are In the case of large-scale power storage, under the situation of widespread use of renewable energy, it is foreseeable that flow batteries will usher in a period of rapid development. At present, the conditions for the general application of flow batteries are not yet available, and in-depth research is still needed on many issues. Cyclic voltammetry tests show that graphite felt has good electrical conductivity, mechanical uniformity, electrochemical activity, acid resistance and strong oxidation resistance, and is a better electrode material. Compared with graphite rods and various powder materials, it is more It is suitable for the research and application of flow batteries. The research shows that the graphite felt electrodes used have been untreated, heat treated and acid heat treated respectively. With the help of a scanning electron microscope, the differences in the surface morphology of the three treatment methods of graphite felt were observed. Heat treatment and acid heat treatment can remove impurities on the surface of graphite felt and pollutants that affect electrochemical reactions, making the surface of graphite felt clean and smooth. The surface condition has been significantly improved. The AC impedance experiment shows that compared with untreated graphite felt, the resistance of graphite felt after heat treatment and acid heat treatment is significantly reduced, which confirms that the activation treatment improves the surface condition of graphite felt, modifies the graphite felt material, and reduces the resistance. Enhanced electrochemical activity
Main material
1. Electrolyte for vanadium batteries
Initially, the electrolyte was prepared by directly dissolving VOSO4 in H2SO4, but due to the high price of VOSO4, people began to turn their attention to other vanadium compounds such as V2O5, NH4VO3, etc. At present, there are two main methods for preparing electrolyte: mixed heating preparation method and electrolysis method. Among them, the mixed heating method is suitable for preparing 1mol/L electrolyte, and the electrolysis method can prepare 3~5mol/L electrolyte.
2. Vanadium battery separator
The diaphragm of the vanadium battery must inhibit the cross-mixing of vanadium ions of different valence states in the positive and negative electrolytes, without hindering the passage of hydrogen ions through the diaphragm to transfer charges. This requires the selection of an ion exchange membrane with good conductivity and better selective permeability, preferably a cation exchange membrane that allows hydrogen ions to pass through. Battery separators are generally based on cation exchange membranes, and Nafion membranes (Dupont) are also available, but the latter is more expensive. One of the ways to improve the efficiency of vanadium batteries is to treat the cation exchange membrane to improve hydrophilicity, selective permeability and increase service life. The perfluorinated sulfonic acid ion exchange membrane was successfully developed by DuPont Company, and it has Nafion as its trademark. It is the ion exchange membrane with the best performance at present.
3. Electrode materials for vanadium batteries
In order to achieve large-capacity energy storage in all-vanadium redox flow batteries, several single cells must be connected in series or in parallel. In this way, except for the terminal electrodes, basically all electrodes are required to be made of bipolar electrodes. Due to the strong oxidizing properties of V02+ and the strong acidity of sulfuric acid, the electrode materials for vanadium batteries must have the characteristics of strong oxidation resistance and strong acidity, low resistance, good electrical conductivity, high mechanical strength, and good electrochemical activity. Vanadium battery electrode materials are mainly divided into three categories: metals, such as Pb, Ti, etc.; carbons, such as graphite, carbon cloth, carbon felt, etc.; composite materials, such as conductive polymers, polymer composites, etc.
Redox flow batteries have many inherent advantages: First, since the output power of redox flow batteries only depends on the size of the battery stack, and the energy storage capacity only depends on the electrolyte storage and concentration, its design is very flexible. When the power is constant, to increase the energy storage capacity, you only need to increase the volume of the electrolyte storage tank or increase the concentration of the electrolyte; secondly, its active material exists in the liquid, so there is no solid phase change and The change of shape, the theoretical life of the active material is long; third, the battery can be discharged at an ultra-deep level (100%) without causing damage to the battery; fourth, the redox flow battery has a large degree of freedom in site selection, occupies less land, and the system operates in a closed system No pollution; Fifth, battery components are mostly cheap carbon materials and engineering plastics, with long service life, rich sources of materials, mature processing technology, and easy recycling.
With the development of the economy, people's demand for energy is getting higher and higher, while fossil energy resources are getting less and less, people will pay more and more attention to the development and utilization of renewable energy such as water energy, wind energy, solar energy and nuclear energy. . From the perspective of stable power supply, energy saving, and CO2 emission reduction, the development and application of efficient new energy storage (electricity) technology has become a top priority. The power density of redox flow batteries is low, but in the field of stationary energy storage, cost and efficiency are the most important. Redox flow batteries have obvious advantages in cost. Compared with lead-acid batteries, their energy efficiency is high, reaching 75% ~ 80%, very cost-effective.


