1. Research Background
Battery safety is critical for Li-ion battery applications, especially high-energy-density batteries for electric vehicles. Silicon, as an anode material with high theoretical specific capacity and abundant reserves, has received extensive attention in lithium-ion batteries. However, the large volume change (300%) of silicon during high-level lithium extraction and intercalation easily leads to particle cracking and pulverization, electrode powder removal, and repeated growth of solid electrolyte interface (SEI) films, resulting in poor cycling stability of silicon anodes. , Coulomb efficiency is low, and even a safety hazard occurs. Therefore, the safety and long cycle performance of silicon anodes, especially under high temperature conditions, are very challenging for high-energy-density Li-ion batteries. Among them, the decomposition of the SEI film is the first step in the thermal runaway of Li-ion batteries, and its thermal behavior is critical to the safety of the battery. Therefore, it is of great significance to systematically study the electrochemical/mechanical/thermochemical behavior of SEI films for high safety and high specific energy lithium-ion batteries.
2. Job introduction
Recently, Hu Xianluo's group from Huazhong University of Science and Technology constructed a robust SEI film on the surface of micron silicon anode through non-flammable ionic liquid-based electrolyte 2 m LiFSI/Pyr14FSI (2 m LiFSI IL), achieving high cycle reversibility and high cycle reversibility of micron silicon anode. Thermal stability, in which micro-silicon is more practical than nano-silicon. The synergistic effect of the SEI film on the safety and reversibility of the micro-Si anode is elucidated for the first time through a comprehensive analysis of the electrochemical, mechanical, and thermochemical behaviors of the micro-Si anode. Benefiting from the heat resistance, high modulus, and rich inorganic components of the SEI film, the silicon anode exhibits excellent cycling performance over a wide temperature range from 25 °C to 80 °C. The enhanced SEI film enables the thermal safety of micro-Si anode-based pouch cells at high temperature to be significantly improved. In addition, advanced characterization techniques also reveal the potential thermal failure mechanism of the micron Si anode, which provides a basis for the material design of highly safe Li-ion batteries with resistance to abuse, high specific energy, and long-cycle performance. The article was published in Small Methods under the title "Interface Engineering to Boost Thermal Safety of Microsized Silicon Anodes in Lithium-Ion Batteries". Liu Qing, a doctoral student at the School of Materials Science and Technology of Huazhong University of Science and Technology, is the first author of this article, and Professor Hu Xianluo is the corresponding author.
3.the core content expression part
3.1 Electrochemical Behavior of Micron Silicon Anode
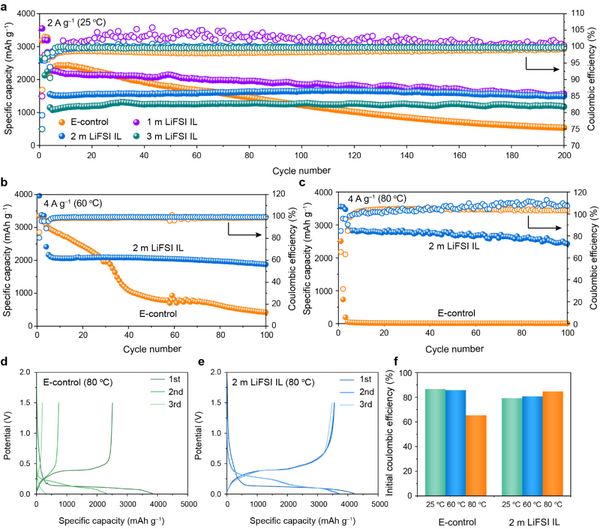
To realize high-performance micro-Si anodes, the electrolyte is the key. The authors optimized and screened the lithium salt concentration of the ionic liquid electrolyte, so that the micro-silicon anode showed excellent electrochemical performance in the designed electrolyte system 2 m LiFSI in Pyr14FSI (2 m LiFSI IL). At room temperature, the micro-Si anode exhibits high specific discharge capacity (1497 mAh g–1) and excellent cycling stability in 2 m LiFSI IL, with a capacity retention rate as high as 96% after 200 cycles (Figure 1a). Compared with graphite anodes, micro-silicon anodes face more severe challenges under high temperature conditions: 1. Huge volume expansion; 2. High temperature makes side reactions aggravated. As shown in Figure 1b, after 100 cycles in 2 m LiFSI IL at 60 °C, the micro-Si anode exhibits a discharge capacity of 1877 mAh g−1 at 4 A g−1, achieving a high capacity retention rate of 86.9%. . Even at a high temperature of 80 °C, the capacity retention rate after 100 cycles is more than 85% (Fig. 1c). Furthermore, compared with the commercial electrolyte (E-control), the micro-Si anode in 2 m LiFSI IL exhibited more stable first Coulomb efficiencies at different temperatures (Fig. 1f). The excellent reversibility and high temperature stability of micro-Si may originate from the formation of a stable SEI film. In order to deeply analyze the stability of micro-Si anodes, the authors further comprehensively analyzed the electrochemical/mechanical/thermochemical behaviors of SEI films and their effects.
3.2 Properties of SEI film
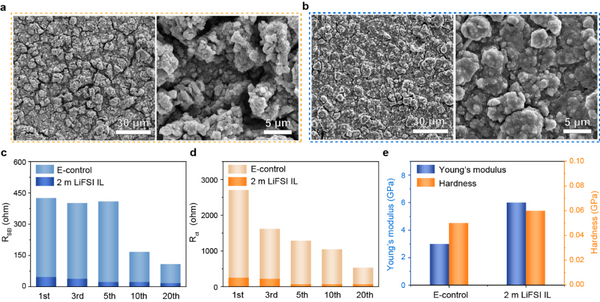
As shown in Fig. 2a-b, clearly different from E-control, after 100 cycles in 2 m LiFSI IL, there is no obvious crack on the surface of the pole piece, so the mechanical integrity and electrical contact of the pole piece are kept well, which is mainly due to Benefit from the formation of a stable SEI film. Furthermore, from the impedance results (Figs. 2c and 2d) of different cycles of cycles, it can be seen that the electrodes in 2 m LiFSI IL exhibited a smaller and more stable RSEI, indicating that the SEI has higher Li+ conductivity and stability. On the contrary, the RSEI and Rct of the electrodes in E-control decreased significantly during the cycling process, which means that the micron silicon particles in the electrodes continued to pulverize, and the formed SEI film could not withstand the huge volume change of the silicon particles. The mechanical properties study of the interfacial SEI film further confirms the superiority of the mid-micron Si anode of 2 m LiFSI IL, as shown in Figure 2e, the average Young's modulus and hardness of the SEI film obtained in 2 m LiFSI IL are higher than E-control. The enhanced Young's modulus and hardness confirm the excellent mechanical properties of the formed SEI films, which are beneficial to maintain the mechanical integrity and electrical contact of the electrodes during cycling.
The changes in the stability and strength of SEI films are mainly attributed to the differences in their composition and structure. Elemental composition analysis by EDX further confirmed the difference in SEI composition (Fig. 3), a thinner and more uniform SEI film was formed in 2 m LiFSI IL, and the content of F, S and N increased, indicating that the formed SEI film was rich in inorganic groups This result is consistent with the mechanical properties and stability of the SEI film.
3.3 Effect of temperature on the surface chemistry of silicon anode

3.3 Effect of temperature on the surface chemistry of silicon anode
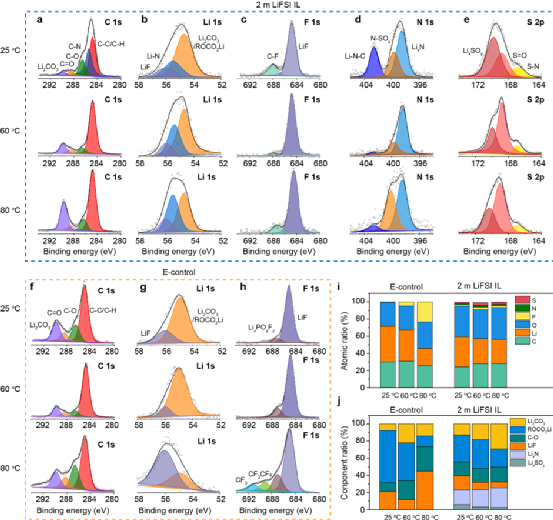
Under high temperature conditions, batteries usually undergo faster ion migration and electrochemical reactions, and the SEI film between the electrode and the electrolyte is more easily decomposed, followed by more severe side reactions, and finally thermal runaway. Therefore, the authors systematically studied the effect of temperature on interface chemistry. influences. As shown in Fig. 4a–e, the SEI film formed on the surface of the micro-Si anode based on 2 m LiFSI IL is rich in inorganic components (LiF and LixN, etc.), and thus has higher mechanical strength and ionic conductivity. In addition, with the increase of temperature, the changes of each element and composition of the SEI film were small (Fig. 4i and 4j). In contrast, in E-control, two new peaks appeared in the F 1s spectrum at 80 °C (Fig. 4h) corresponding to CF2CF2 and CF3, respectively, and the F content in the SEI film at 60 °C and 80 °C significantly increased (Fig. 4i), indicating that LiPF6 decomposes severely under high temperature conditions and undergoes violent side reactions with the solvent. The above results indicate a highly stable interfacial chemical reaction between the micron anode and 2 m LiFSI IL at high temperature.
3.4 Intrinsic thermal stability of SEI films
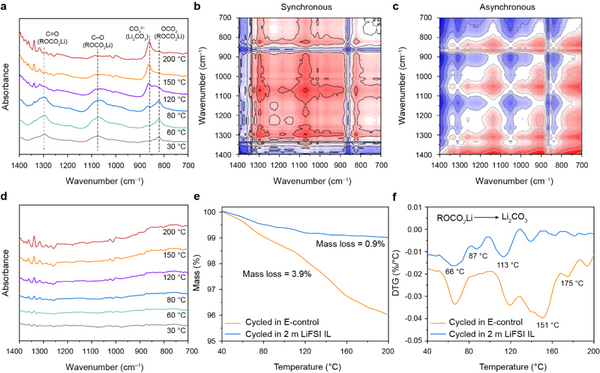
The intrinsic thermal stability of SEI films is critical for the electrochemical performance and safety of silicon-based batteries. The authors first compared the variable temperature Fourier transform infrared spectra of the pole pieces after cycling in E-control and 2 m LiFSI IL. As shown in Fig. 5a, at 30 °C, the FTIR spectrum of the silicon anode in E-control showed three v (C=O), v (C-O) and v (OCO2) at 1295, 1074 and 823 cm-1, respectively. There are obvious peaks, indicating that there are abundant organic components in the SE film, which is consistent with the XPS results. With the increase of temperature, the above three peaks gradually weakened and almost disappeared around 150 °C. Meanwhile, a new peak v(CO32–) corresponding to Li2CO3 appeared at 862 cm-1, and its intensity increased with increasing temperature. The above results show that the poor thermal resistance of the organic components of the SEI film formed in E-control leads to the poor intrinsic thermal stability of the SEI film. In contrast, the surface of the Si anode cycled in 2 m LiFSI IL has few peaks of organic components (Fig. 5d), indicating that the SEI film is dominated by inorganic components. In addition, the change of the FTIR spectrum is hardly affected by temperature, further verifying the intrinsic thermal stability of the SEI film. In addition, the TG results of the silicon anode after cycling further verified the above conclusion.
The intrinsic thermal stability of SEI films is critical for the electrochemical performance and safety of silicon-based batteries. The authors first compared the variable temperature Fourier transform infrared spectra of the pole pieces after cycling in E-control and 2 m LiFSI IL. As shown in Fig. 5a, at 30 °C, the FTIR spectrum of the silicon anode in E-control showed three v (C=O), v (C-O) and v (OCO2) at 1295, 1074 and 823 cm-1, respectively. There are obvious peaks, indicating that there are abundant organic components in the SE film, which is consistent with the XPS results. With the increase of temperature, the above three peaks gradually weakened and almost disappeared around 150 °C. Meanwhile, a new peak v(CO32–) corresponding to Li2CO3 appeared at 862 cm-1, and its intensity increased with increasing temperature. The above results show that the poor thermal resistance of the organic components of the SEI film formed in E-control leads to the poor intrinsic thermal stability of the SEI film. In contrast, the surface of the Si anode cycled in 2 m LiFSI IL has few peaks of organic components (Fig. 5d), indicating that the SEI film is dominated by inorganic components. In addition, the change of the FTIR spectrum is hardly affected by temperature, further verifying the intrinsic thermal stability of the SEI film. In addition, the TG results of the silicon anode after cycling further verified the above conclusion.
3.5 Thermal runaway characteristics and mechanism analysis
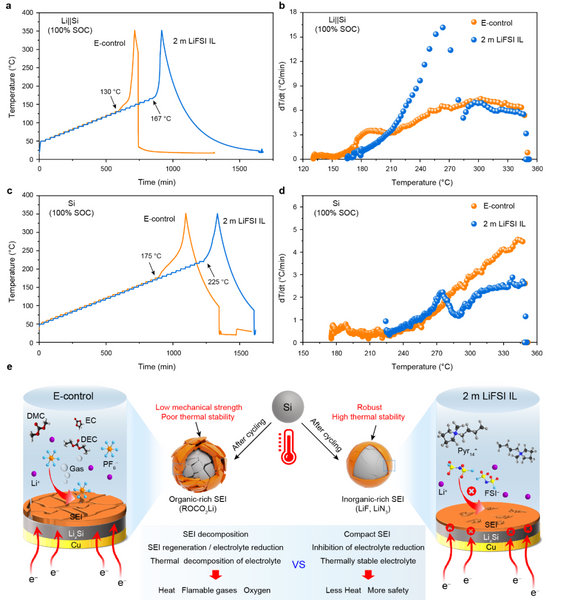
To investigate the effect of SEI films on the thermal runaway characteristics of batteries, the authors systematically compared the thermal runaway processes of pouch batteries under different conditions by means of an acceleration calorimeter (ARC). The Tonset values of Li||Si pouch cells with electrolytes of E-control and 2 m LiFSI IL 100% SOC were 130 °C and 167 °C, respectively (Fig. 6a), indicating that 2 m LiFSI compared with E-control IL can delay thermal runaway, and the improvement of thermal stability of Li||Si pouch cells by 2 m LiFSI IL electrolyte may originate from the SEI film with high thermal stability. To further eliminate the interference of the Li foil, it was removed from the fully lithiated Li||Si pouch cell and assembled into a new pouch cell (Si, 100% SOC). As shown in Figure 6c, the Tonset value of the pouch cell containing micron silicon-based (100% SOC) in 2 m LiFSI IL reaches 225 °C, which is much higher than that of the cell with E-control. Furthermore, the distribution of the temperature rise rate (Fig. 6d) is consistent with the above results, indicating that the SEI formed in the 2 m LiFSI IL effectively suppresses the thermal runaway of the microscale Si electrodes. For the 2 m LiFSI IL, the SEI film produced by the electrochemical reaction at the interface is rich in high-temperature-resistant inorganic components and thus is stable over a wide temperature range (Fig. 6e). In addition, the inorganic component-rich SEI film has sufficient mechanical strength to withstand the volume expansion of silicon particles. Therefore, the SEI film, interfacial electrochemistry, thermochemistry, and nanomechanics need to be taken into account when designing a safe micron-silicon-based high-specific-energy lithium-ion battery.
To investigate the effect of SEI films on the thermal runaway characteristics of batteries, the authors systematically compared the thermal runaway processes of pouch batteries under different conditions by means of an acceleration calorimeter (ARC). The Tonset values of Li||Si pouch cells with electrolytes of E-control and 2 m LiFSI IL 100% SOC were 130 °C and 167 °C, respectively (Fig. 6a), indicating that 2 m LiFSI compared with E-control IL can delay thermal runaway, and the improvement of thermal stability of Li||Si pouch cells by 2 m LiFSI IL electrolyte may originate from the SEI film with high thermal stability. To further eliminate the interference of the Li foil, it was removed from the fully lithiated Li||Si pouch cell and assembled into a new pouch cell (Si, 100% SOC). As shown in Figure 6c, the Tonset value of the pouch cell containing micron silicon-based (100% SOC) in 2 m LiFSI IL reaches 225 °C, which is much higher than that of the cell with E-control. Furthermore, the distribution of the temperature rise rate (Fig. 6d) is consistent with the above results, indicating that the SEI formed in the 2 m LiFSI IL effectively suppresses the thermal runaway of the microscale Si electrodes. For the 2 m LiFSI IL, the SEI film produced by the electrochemical reaction at the interface is rich in high-temperature-resistant inorganic components and thus is stable over a wide temperature range (Fig. 6e). In addition, the inorganic component-rich SEI film has sufficient mechanical strength to withstand the volume expansion of silicon particles. Therefore, the SEI film, interfacial electrochemistry, thermochemistry, and nanomechanics need to be taken into account when designing a safe micron-silicon-based high-specific-energy lithium-ion battery.
Summary
Through screening and design, the authors found that a medium-concentration ionic liquid-based electrolyte has good compatibility with micron silicon anodes, and can form SEI films with thermal stability, high modulus and rich inorganic components. Soft pack battery high thermal safety. This work systematically investigates the formation of SEI films and their associated thermochemical/electrochemical/nanomechanical behaviors, helping to elucidate the thermal failure mechanism of microscale silicon anodes and providing important insights into the rational design and development of intrinsically stable/safe electrodes. Learn and reference.
Through screening and design, the authors found that a medium-concentration ionic liquid-based electrolyte has good compatibility with micron silicon anodes, and can form SEI films with thermal stability, high modulus and rich inorganic components. Soft pack battery high thermal safety. This work systematically investigates the formation of SEI films and their associated thermochemical/electrochemical/nanomechanical behaviors, helping to elucidate the thermal failure mechanism of microscale silicon anodes and providing important insights into the rational design and development of intrinsically stable/safe electrodes. Learn and reference.

