Due to its abundant resources and low cost, sodium-ion batteries are very suitable for short-distance electric vehicles and large-scale energy storage. Compared with lithium-ion batteries, the molar mass of sodium ions as charge carriers is larger, resulting in lower energy density of sodium-ion batteries under the same material system.
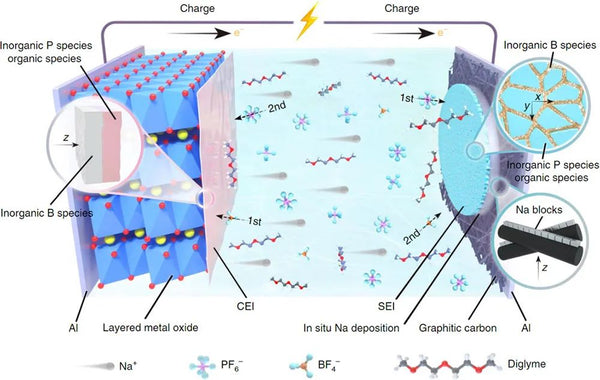
Using metallic sodium with high theoretical specific capacity (1166 mAh/g) as the anode is an effective way to improve the energy density of Na-ion batteries. However, the air stability of sodium metal is poor, which poses a great safety hazard in the mass production of batteries, and the texture of sodium metal is soft and sticky, making it difficult to make an ultra-thin negative electrode, resulting in the sacrifice of excess active material on energy density. In this context, the strategy of electrochemically in-situ formation of a sodium anode on the collector side of the anode using the sodium ions extracted from the cathode during the first cycle of charging can effectively solve the problems of metal sodium air sensitivity and ultrathinization difficulties. However, the unstable cathode and anode interface will continuously consume limited sodium ions, and in the process of repeated electroplating and stripping, the uneven deposition of sodium ions will also lead to the generation of "dead sodium" or sodium dendrites, thereby reducing the coulombic efficiency, leading to rapid capacity decay.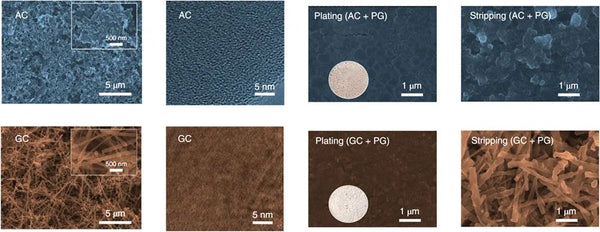 To solve the above problems, Li Yuqi, a doctoral student at the Institute of Physics, Chinese Academy of Sciences/Beijing National Research Center for Condensed Matter Physics, under the guidance of Researcher Hu Yongsheng and Associate Researcher Lu Yaxiang, coordinated the current collector/sodium, sodium/electrolyte (SEI) and electrolyte/ Positive electrode (CEI) triple interface, reducing the size of sodium nucleation, repairing sodium deposition cracks, effectively inhibiting the growth of sodium dendrites and preventing the dissolution of transition metal ions such as iron and nickel, achieving energy density without additional sodium source supplementation Ah-grade sodium-ion batteries up to 205 Wh/kg. The energy density of this sodium-ion battery system is even better than that of the current commercial lithium iron phosphate||graphite battery system.
To solve the above problems, Li Yuqi, a doctoral student at the Institute of Physics, Chinese Academy of Sciences/Beijing National Research Center for Condensed Matter Physics, under the guidance of Researcher Hu Yongsheng and Associate Researcher Lu Yaxiang, coordinated the current collector/sodium, sodium/electrolyte (SEI) and electrolyte/ Positive electrode (CEI) triple interface, reducing the size of sodium nucleation, repairing sodium deposition cracks, effectively inhibiting the growth of sodium dendrites and preventing the dissolution of transition metal ions such as iron and nickel, achieving energy density without additional sodium source supplementation Ah-grade sodium-ion batteries up to 205 Wh/kg. The energy density of this sodium-ion battery system is even better than that of the current commercial lithium iron phosphate||graphite battery system.
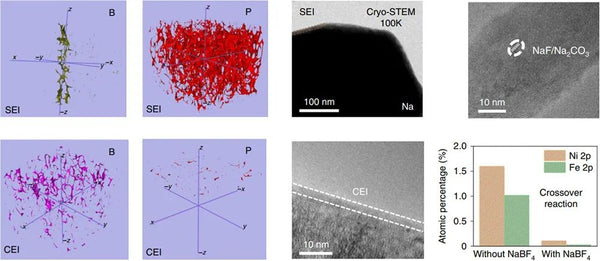
The key to achieving high specific energy and long cycle life of this type of battery lies in the comprehensive design of the cathode, current collector and electrolyte side. On the positive side, this study selected a cobalt-free copper-based O3 phase layered oxide to ensure the high energy density of the battery while being low-cost and sustainable. In order to achieve a smoother deposition of metal sodium from bottom to top, a graphitized carbon coating is selected on the negative current collector side to make sodium ions have a low nucleation overpotential, and control the uniform and dense nucleation of metal sodium in a smaller size. However, the study found that even with the sodium-philic current collector coating, there are still cracks on the surface of the metal sodium deposited in the traditional ether electrolyte. Uniform sodium deposition and even sodium dendrite formation. In order to further solve the above-mentioned interfacial cracking problem, a boron salt-containing electrolyte was designed in this study, and its decomposition products were captured by multi-scale interface characterization techniques. In addition, the boron-oxygen component exhibits a two-dimensional distribution in the outer layer of the interface film on the negative electrode side, and a three-dimensional distribution in the inner layer of the interface film on the positive electrode side. Based on quantum chemical/molecular dynamics simulations, it is found that the electrolyte after adding boron salt has a special solvation configuration which affects the decomposition order on the positive and negative sides, which reasonably explains the boron-containing components on the positive and negative sides. Strange phenomena of heterogeneous distributions. The strong and flexible SEI film effectively inhibits the formation of dead sodium and sodium dendrites and repairs the cracks formed during the deposition and stripping of metallic sodium, while the ultrathin CEI film protects the structural integrity of the cathode and hinders transition metals. ions dissolve.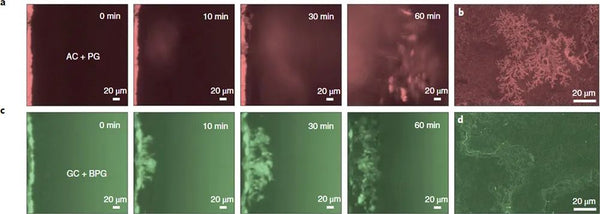
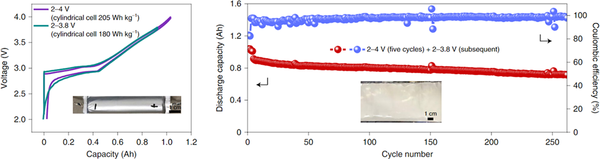
Benefiting from the above-mentioned triple synergistic interface (current collector/sodium interface, sodium/electrolyte interface, and electrolyte/cathode interface) effect, highly reversible metallic sodium deposition (rich metallic luster) is achieved, and the assembled Ah-grade cylinders Type sodium-ion batteries have a cycle life of up to 260 times without applying additional pressure and without high temperature cycling. Insights from this work on intrinsic Na deposition/stripping behavior (different from Li metal deposition) and interfacial chemistry provide valuable insights into the further development of higher-performance Na-ion batteries.
 This work was supported by the Strategic Pilot Science and Technology Project of the Chinese Academy of Sciences (XDA21070500), the National Natural Science Foundation of China (51725206, 52122214, 52072403), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2020006), and the Beijing Natural Science Foundation (2212022). The related results, titled "Interfacial engineering to achieve an energy density of over 200 Wh kg-1 in sodium batteries", were published on Nature Energy on June 2, 2022 (https://doi.org/10.1038/s41560- 022-01033-6).
This work was supported by the Strategic Pilot Science and Technology Project of the Chinese Academy of Sciences (XDA21070500), the National Natural Science Foundation of China (51725206, 52122214, 52072403), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2020006), and the Beijing Natural Science Foundation (2212022). The related results, titled "Interfacial engineering to achieve an energy density of over 200 Wh kg-1 in sodium batteries", were published on Nature Energy on June 2, 2022 (https://doi.org/10.1038/s41560- 022-01033-6).

