What happens if lead acid battery runs out of water?
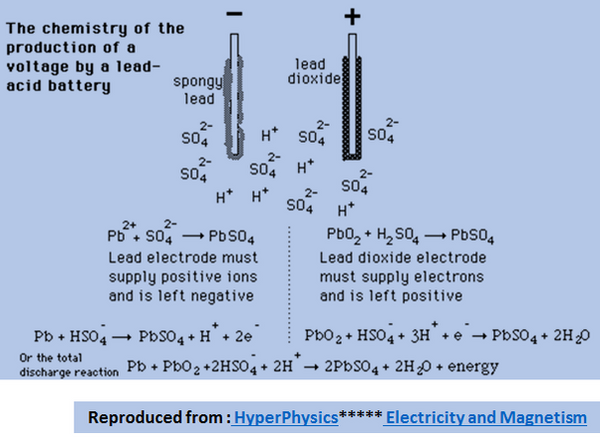
A Lead Acid Battery is constructed with Sponge Lead as its Cathode and Lead Dioxide as its Anode. The electrochemical Cell and the reactions at the Anode and Cathode are illustrated below.
The overall reactions which is a Reversible Reaction is written as :

The forward reaction indicates that the acid is used and more of Salt (Lead Sulphate) and Water is formed. Please note that the site of this reaction is in the Cathode and with the flow of the electrons in the outer circuit, the reaction at the Anode takes place with the dissolution of the Lead Oxide. In the process the electrons produced can be used to do work. (Discharging the Battery)
The backward reaction indicates that the Water and the Lead Salt (Lead Sulphate) reacts to form the Acid, Lead Metal and Lead Oxide. Please note that the site of this reaction is in the Anode and with the formation of the salt, the reaction at the Cathode progresses to form the acid and Metallic Lead regenerated again. However, for this reaction, electrons need tobe pumped into the system. (Recharging the Battery)
Voltage of the Cell remains constant at around 2V per cell for quite some-time in a fully charged battery, but gets to 1.65 on continuous discharge and towards the tail end of the discharge cycle.
The Sulphuric Acid used in the lead acid batteries are at an average concentration of 40%. It could vary marginally by 5% either way, from one manufacturer to other. At this concentration, the specific gravity of the acid is around 1.3. When the battery is fully discharged the specific gravity measures around 1.15, and at this stage, you shall get a voltage of 1.6V. The table below gives a relationship of charge to the density of the acid.
1.300-1.280--Fully charged.
1.280-1.200--More than half charged.
1.200-1.150--Less than half charged.
1.150 and less--Completely discharged.
However, the currentproduced is dependent on the factor : the quantum of the reaction, which againis guided by two factors :
1) the Concentration of the Acid.
2) The surface area of the Cathode and Anode.
While the Voltage remains the same at 12 V, a Car Battery produces around 36AH of current, and a 100AH for bigger trucks. This is achieved by either increasing the number of cells, connected in Parallel, or making the cells larger to produce more chemical reaction.
For this cell to be effective, and to work at this Constant Voltage of 2V with the rated current of the cell, the concentration of Sulphuric Acid is of paramount importance. What will happen when the acid is dilute is what we have seen above.
What will happen if we remove the water (Due to a Battery which has been kept open or we did not add any distilled water after fully discharging it)?
1) As there is no water, the formation of salts has progressed to completion without any water for dissolving them. This means that there is no formation of ions and no transporters of electric current inside the cells and hence there is no possible conduction.
2) If you recharge the battery now, it is going to become hot. Either the battery is going to explode or catch fire, as you are now pumping electricity into the battery for charging, without any electrolytes to carry the current inside the battery between the Anode and Cathode. Now you are inviting danger, with a sure recipe for disaster.
Removing water in a lead acid battery is normally the result of overcharging, breaking water in the electrolyte into hydrogen and oxygen gasses that are expelled through its venting system. The concentration of sulfuric acid in the electrolyte (typically 38%) naturally increases with further overcharging. This often happens if a charging system voltage regulator goes bad and charging continues at a high voltage.
As water is consumed, the electrolyte concentration increases and the electrolyte level in the battery drops with the loss of water volume. Eventually it falls below the top of the electrodes exposing them to oxygen from the atmosphere and charging gasses. The porous lead in the section of negative electrode exposed will react with the oxygen and sulfuric acid wicked from the electrolyte to produce lead sulfate that removes some further volume from the electrolyte, but reduces its concentration or prevents it from continuing to increase, depending on the charge rate.
If charging is continued, the battery will eventually become virtually dry, but the electrolyte will also not be extremely high concentration because the sulfate becomes tied up in the negative electrode. Surprisingly, the battery will perform limited starting capability even when the electrolyte is very low because the typically used microporous separators remain saturated and conductive to ionic current.
If not allowed to sit for a long time in this condition and if no other damage has been caused by overcharging, refilling the battery with water followed by a slow recharge may bring the battery back to life, but obviously not to its original condition.
Thus, during a typical dry out scenario where current is not excessive on a continuous basis in service, the electrolyte concentration in a lead acid battery will never exceed a certain concentration and will never approach the concentration of concentrated sulfuric acid. There will always be a certain amount of water in the electrolyte even when the battery appears "dry".
JUNLEE Group is an integrated full power energy factory that specializes in Uninterruptible Power Supply (UPS), Lead-Acid Battery, Battery pack, EV battery, Energy Storage Battery, Energy storage power station, Power pack Gel battery, PV Inverter and Solar system.
Production capacity reach 200000 KVaH per month. Products apply to Electric vehicles,electric mobility, solar & wind energy storage system, UPS, backup power, telecommunication, medical equipment and lighting.
JUNLEE sets up "Power research center" with more High-tech products.More than 100 engineers provided in-time and efficient one-stop solutions.
They mission strives to bring green power to the world.
To learn more about Li-ion batteries, please refer to https://www.junleepower.com/

