【Research Background】
The long battery life and fast charging of electric vehicles require high-performance lithium-ion batteries, and the cathode material is one of the most important components. However, the positive electrode is easily broken during cycling, and there is a continuous side reaction with the electrolyte, which seriously damages the cycle life and rate performance of the battery. Surface coating can reduce stress, increase wettability of liquid electrolyte and reduce interfacial charge transfer resistance, reduce side reactions, thereby effectively optimizing cathode materials. However, the influence of the physicochemical properties of surface coatings on the electrochemical performance and the evolution rules during cycling still need to be further understood. In addition, the best surface coating materials and coating methods have not yet been systematically summarized and summarized.
【Job description】
Ilias Belharouaka et al. from Oak Ridge National Laboratory in the United States published a review article summarizing the chemical and physical properties and selection criteria of cathode coatings. In addition, the concept of coating thickness and the method to achieve uniform coating are discussed, and the latest progress, effectiveness and necessity of positive electrode surface coating are summarized, and the relationship between coating type/thickness and lithium ion diffusion through the coating layer is pointed out. Correlations between structural properties. The relevant research results were published in the top international journal Energy Storage Materials under the title of "Valuation of Surface Coatings in High-Energy Density Lithium-ion Battery Cathode Materials".
【Details】
1. Requirements for positive electrode surface coating
Requirements for surface coatings include: 1) thin and uniform; 2) ionic and electronic conductivity; 3) high mechanical properties and stability after charge/discharge cycles; 4) facile and scalable coating process.
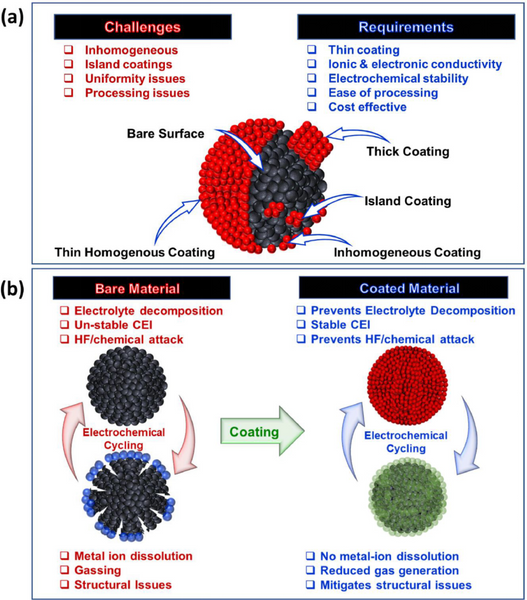
2. The role of surface coating
The surface coating effect of positive electrode materials: 1) Physical barrier, inhibiting side reactions; 2) Clearing HF, preventing chemical corrosion of electrolytes, and reducing transition metal dissolution; 3) Improving electronic and ion conduction; 4) Surface chemical modification, promoting interface ions Charge transfer; 5) Stabilize the structure and relieve the phase transition stress.
3. Coating structure/morphology
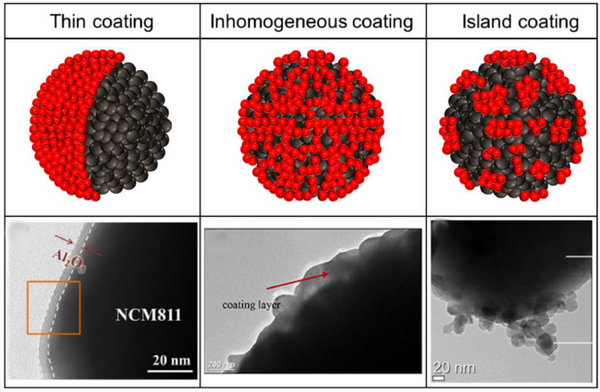
3.1. Uniform and thin coating
The cladding should be uniform and thin. The complete coverage of the cathode particles will protect the cathode from electrolyte attack and suppress side reactions. In addition, thin coatings can enhance the kinetics at the interface and improve battery performance.
3.2, thick cladding
The thick coating provides a good physical barrier between the cathode and the electrolyte. However, a thicker coating hinders the diffusion of lithium during deintercalation, which may have a good effect in high temperature operation.
3.3. Island/rough cladding
It is difficult to obtain an even and thin coating over the entire material using dry and wet coating processes. The coatings formed by these processes are rough and uneven.
4. Coating process/strategy
4.1. Wet process
4.1.1. Sol-gel coating
The sol-gel coating process is usually used for the synthesis of cathode materials and surface coating. However, costs are increased due to the use of water or other solvents. In addition, solvents such as water can cause lithium leaching and change the cathode surface stoichiometry.
4.1.2. Hydrothermal/Solvent Thermal Coating
The cladding layer developed by the hydrothermal/solvothermal process is nanoscale and uniform, and the stoichiometry of the cladding layer can be controlled. However, it is difficult to process, the precursor salt is expensive and the yield is low.
4.2. Dry coating process
Dry coating is probably the most feasible and suitable method, however it is difficult to obtain a uniform coating.
4.3. Gas-phase chemical process
4.3.1. Chemical Vapor Deposition (CVD)
Chemical Vapor Deposition (CVD): At a certain temperature, the reactants decompose on the substrate material, allowing the material to be deposited from the vapor phase. The main advantage of CVD is that it can produce low porosity, uniform and thin coatings.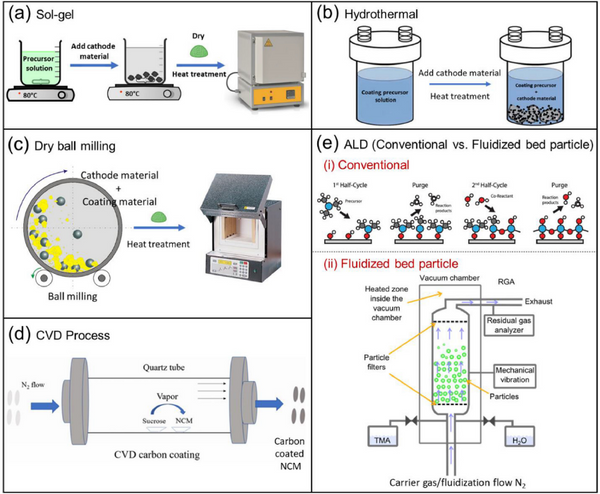
4.3.2. Atomic Layer Deposition (ALD)
The cladding layer formed by atomic layer deposition (ALD) is atomically thick. Its biggest advantage is that it can form a uniform, high-quality cladding layer with precise control, but it has low yield, slow processing time, high cost of precursor, toxicity and complicated process.
5. Type of cladding material
5.1. Metal oxides
The metal oxide coating acts as a physical barrier between the positive electrode material and the electrolyte, and does not participate in the electrochemical reaction. The disadvantage is that the lithium ion conductivity is poor. In some cases, the rate performance of metal oxide-coated cathode materials is reduced. This is due to an increase in impedance (Rct). However, there are few reports that such inert metal oxide coating can improve charge transfer.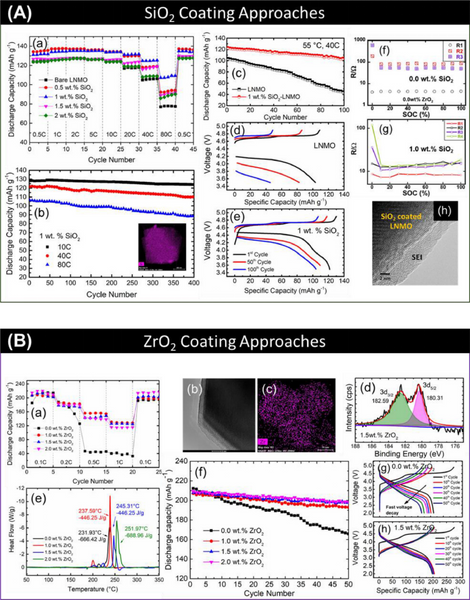
5.2. Phosphate
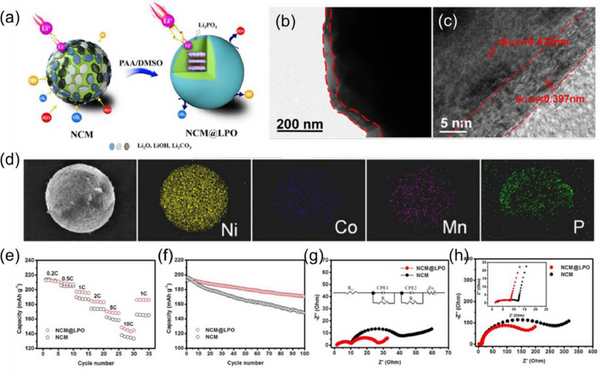
Phosphate coating can improve the ion transport performance of cathode materials. Poor recycling and safety concerns of nickel-rich layered oxides hinder their large-scale use. Surface coating is an effective way to alleviate the challenges of nickel-rich cathodes. The Li3PO4 coating on the NCM surface prevents the direct contact between the NCM cathode surface and the electrolyte, thereby suppressing the side reactions and the formation of resistive surface films.
5.3. Electrode material as coating
Electrode materials have been used as cathode cladding materials. Generally, more stable materials should be coated over less stable materials to improve the overall stability and performance of the material. The benefit is that they provide a physical barrier between the cathode and the electrolyte, suppress side reactions, improve charge transfer kinetics, and enable cathode materials with better electrochemical performance. However, it is difficult to achieve uniform and thin electrode material coating. Moreover, a high heat treatment temperature is required to form a good coating, which may lead to the decomposition of the cathode material. For this type of cladding, it is necessary to select the best cladding material and cladding conditions. For example, lithium-rich Li1.2Mn0.6Ni0.2O2 layered oxide (USMLLR) coated with ultrathin spinel (LiMn2O4) has improved electrochemical and thermal performance. The advantage is that it not only ensures the high capacity of Li-rich layered oxide materials but also provides high rate performance, and at the same time improves the charge transport at the surface due to the excellent Li+ conductivity of LMO.
5.4. Solid electrolytes and other ionic conductors as coatings
Solid electrolytes have high ionic conductivity at room temperature and are suitable as cathode coatings, but have low electronic conductivity. Due to their high ionic conductivity, they are expected to improve charge transfer at the cathode/electrolyte interface. In addition, the solid electrolyte coating provides a physical barrier that suppresses side reactions. Coating lithium lanthanum titanate (LLTO) on LiNi0.6Co0.2Mn0.2O2 (NCM) can improve the rate performance, which is attributed to the high ionic conductivity of the LLTO coating and the suppression of side reactions. However, an increase in cladding thickness can inhibit the electron transfer process during charge/discharge.
5.5. Conductive polymers
The conductive polymer coating can form a uniform film with high electronic conductivity, improving charge transfer at the cathode/electrolyte interface. Such polymers can accommodate volume changes and reduce crack formation. Figure 8 shows PANI-PEG coated LiNi0.8Co0.1Mn0.1O2 (NCM-811). PANI-PEG has high ionic and electronic conductivity. The enhanced rate capability can be attributed to the enhanced electronic conductivity and charge transfer. Cycling performance is also improved due to the suppression of side reactions.
5.6. Surface doping
The surface coating method is to form a physical barrier layer on the surface of the positive electrode, which is usually less reactive to the electrolyte, thus improving the structure and thermal stability of the material. Since the crystal structure and the composition changes at the interface are similar, the surface doping will not hinder the diffusion of Li+, which reduces the Rct and the mechanical stress at the interface, and reduces the possibility of cracking. Figure 9 shows a nickel-rich cathode doped with manganese surface. The Mn-rich layer effectively stabilizes the Ni-rich surface by suppressing side reactions, ultimately improving thermal, structural, and electrochemical performance. The newly formed surface is highly stable while maintaining high ion transport properties
6. Structure-Property Correlation: Coating Thickness and Li-ion Diffusion
Certain surface coatings hinder ion diffusion but provide other advantages, while some coatings enhance ion diffusion at the expense of other properties. The trade-off between these effects has been a focus of battery research when encapsulation is employed. The structural-property correlation between cladding thickness and Li-ion diffusivity of the cladding layer is an effective measure of the trade-off criteria. Figure 10 provides diffusion time versus cladding thickness. Most oxides and fluorides have extremely low lithium ion diffusivity. For thicker coatings, the diffusion time is longer. The thickness of these surface coatings should be less than 10 nm for better electrochemical performance.
picture
Figure 10. Comparing the diffusion time of different cladding materials at 300 K versus cladding thickness
7. All-solid-state battery cathode material coating
7.1. Necessity and function of positive electrode coating in all-solid-state batteries (SSB)
All-solid-state batteries are considered to be the focus of battery research and development due to their high safety. However, there is an issue of instability when the solid electrolyte (SE) is in direct contact with the cathode material. When in direct contact with cathode materials, SE is easily oxidized, forming a resistive interface and increasing the battery impedance, resulting in rapid capacity fading. Surface coating of cathode materials is also required in SSBs to suppress these oxidation reactions. Therefore, SSB cathode materials require an electrically insulating and ionically conductive surface coating.
Inorganic SEs can be classified into three major categories: oxides, sulfides, and argentites. Compared with oxide SEs, sulfide SEs have extremely high ionic conductivity, but are less chemically stable and have a narrower voltage window, resulting in limited battery energy densities. In addition, when in direct contact with cathode materials, sulfide SEs form a resistive interface and the interfacial resistance increases.
7.2. Challenges of Cathode Coating in SSB
The coating layer should cover the cathode particles uniformly and completely, and ensure the contact between the cathode particles to provide a conductive network. Hard and brittle coatings are prone to crack formation during electrode fabrication, thus forming particle contacts, but may lead to SE decomposition. If the cladding is plastic and deformable, it can be deformed to form new contact points without crack formation. The surface coating should suppress or accommodate the volume change of the cathode material to maintain the interparticle contact. In addition, the interdiffusion between cathode active material and SE may also occur. Coating suppresses this interdiffusion, mitigating the formation of resistive interfaces.
In summary, the cathode coating in SSB should have the following properties: 1) provide a physical barrier to prevent side reactions; 2) prevent interdiffusion and suppress the formation of resistive interfaces; 3) have low electronic conductivity to avoid redox reactions of SE; 4) High ionic conductivity, improving charge transfer at the interface; 5) Good mechanical properties, adapting to the volume change of positive electrode materials and preventing contact loss; 6) Reducing interface resistance

